SAFETY PROFILE (ADVERSE REACTIONS) IN CLINICAL TRIALS OF BANZEL® (rufinamide)
SAFETY PROFILE (ADVERSE REACTIONS) IN CLINICAL TRIALS OF BANZEL®
The safety of BANZEL® has been evaluated in children and adults
Safety evaluated in children
In pediatric patients with epilepsy treated in all double-blind, adjunctive clinical trials (at recommended dose of 45 mg/kg/day)1
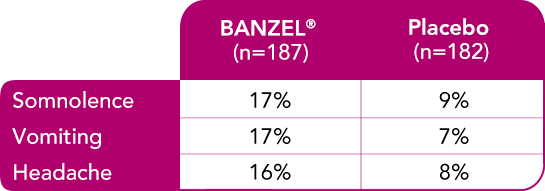
Most common treatment-emergent adverse reactions (≥10%) with a higher frequency than placebo

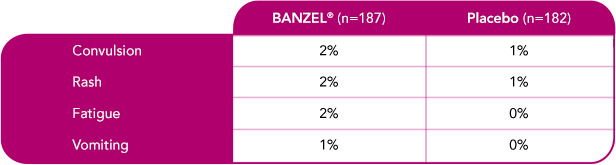
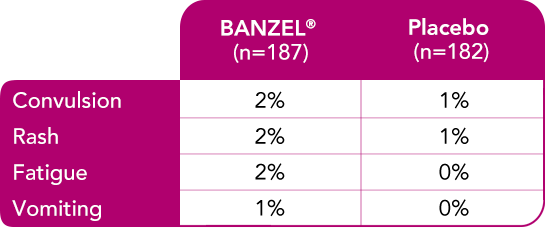
Most common treatment-emergent adverse reactions (>1%) leading to discontinuation
Pediatric (age 1 to less than 4 years)
- In pediatric patients (1 year to less than 4 years of age) with Lennox-Gastaut syndrome, the most commonly observed (≥10%) adverse reactions and with a higher frequency with BANZEL vs any other AED, respectively, were vomiting (24% vs 9%), somnolence (16% vs 0%), constipation (12% vs 9%), cough (12% vs 9%), bronchitis (12% vs 0%), rash (12% vs 9%), and decreased appetite (12% vs 9%)2
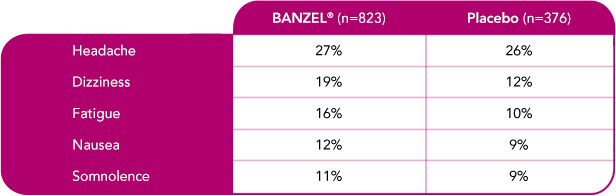
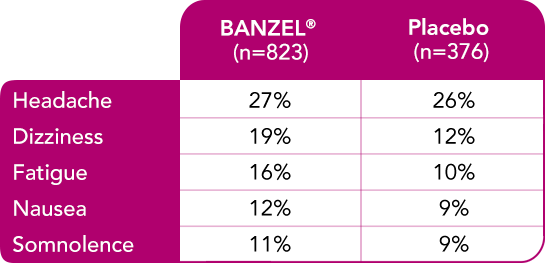
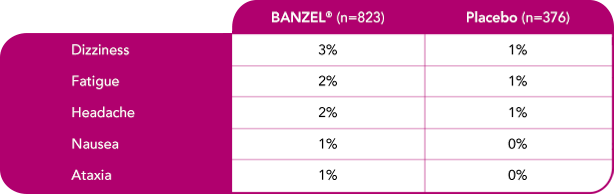
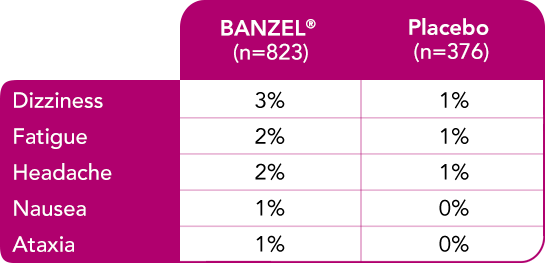
Safety evaluated in adults
In adult patients with epilepsy treated in all double-blind, adjunctive clinical trials (at doses up to maximum dose of 3200 mg/day)1
Most common treatment-emergent adverse reactions (≥10%) with a higher frequency than placebo
Most common treatment-emergent adverse reactions (>1%) leading to discontinuation
- References: 1. BANZEL® (rufinamide) prescribing information, Eisai Inc. 2. Data on file, Eisai Inc.