TRIAL PATIENTS HAD HISTORY OF MULTIPLE SEIZURE TYPES, UNCONTROLLED ON 1-3 AEDs1-3
Trial design1,3
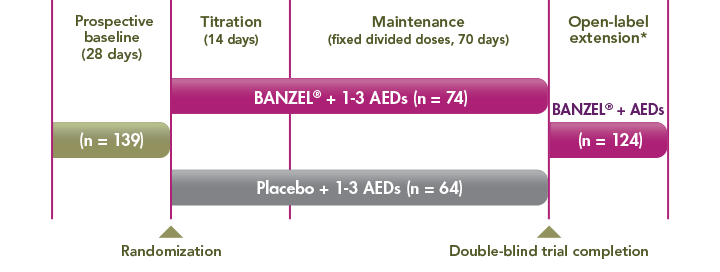
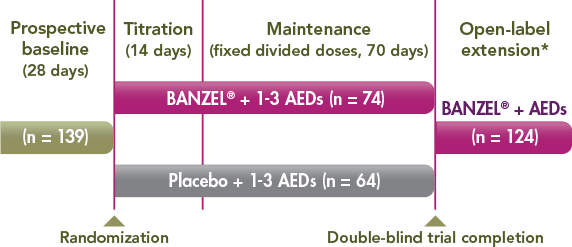
*Approximately 3 years beginning with a 2-week, double-blind conversion phase.
- A 12-week, randomized, double-blind, multicenter, placebo-controlled, parallel-group trial to assess the effectiveness of BANZEL (rufinamide) to reduce inadequately controlled seizures associated with LGS in patients (N=138, intent to treat) being treated with 1-3 concomitant stable-dose AEDs1-3
- The primary efficacy variables were the percent change in total seizure frequency per 28 days, the percent change in tonic-atonic (drop attacks) seizure frequency per 28 days, and the seizure severity rating from the parent/guardian global evaluation of the patient's condition1
- All 3 primary endpoints met the prespecified statistical criteria for effectiveness1
BANZEL® trial population: key inclusion criteria†1,3

seizures in month prior to baseline

History of multiple seizure types
History of tonic-atonic and atypical absence seizures

Inadequately controlled with 1-3 AEDs
†Presents a partial list of criteria; there were additional inclusion and exclusion criteria in the pivotal trial.
Median number of total seizures in the 2 treatment groups during the 28-day baseline phase1:
BANZEL ® pivotal trial entry criteria
Patients in the BANZEL trial were subject to the following entry criteria ‡
INCLUSION
EXCLUSION
INCLUSION
EXCLUSION
‡This is not the complete list of inclusion/exclusion criteria.
-
Baseline characteristics of BANZEL study participants were similar
between study groups (BANZEL vs placebo):
- Gender ( 62.2% male vs 62.5% male), median age (13 years vs 10.5 years), median duration of LGS (8 years vs 8 years), and median weight (36 kg vs 34 kg)
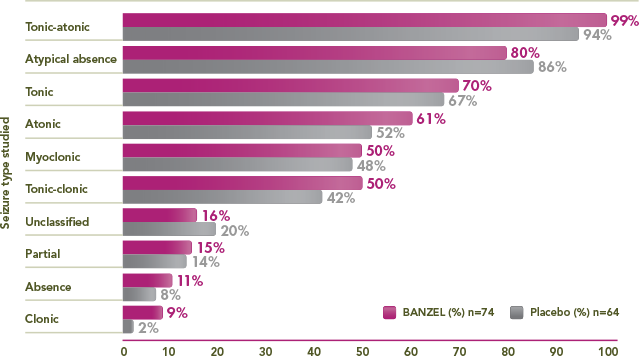
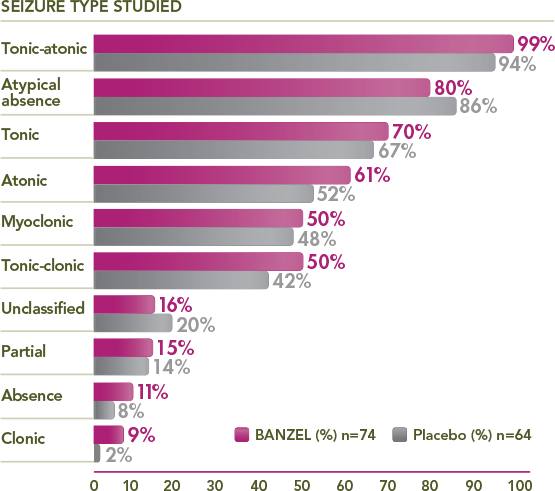
Multiple seizure types observed at baseline
≥50% of BANZEL patients presented with tonic-atonic, atypical absence, tonic, atonic, myoclonic, and/or tonic-clonic seizures
Percentage of trial patients with specific seizures at baseline1,3
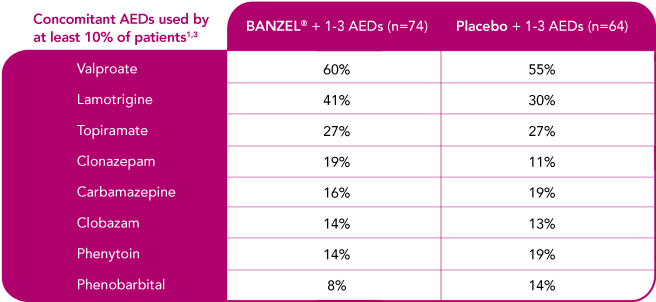
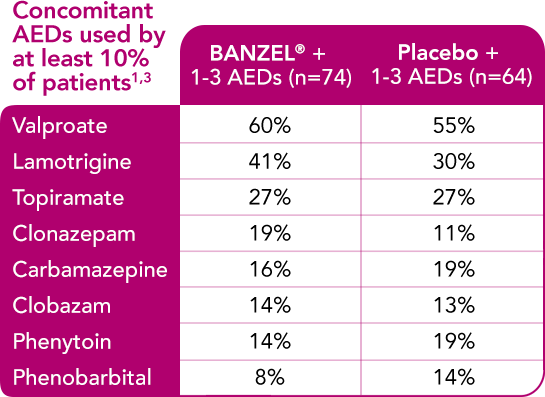
BANZEL® was studied as adjunctive treatment with a range of AEDs
Concomitant AEDs in the BANZEL® clinical trial
- References: 1. Glauser et al. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology. 2008;70(21):1950-1958. 2. BANZEL® (rufinamide) prescribing information, Eisai Inc. 3. Data on file, Eisai Inc.


